9/12/2025
by Tracy TreDenick
BioTechLogic Co-Founder & Head of Regulatory and Quality
Download this paper in PDF
Early-stage biopharma companies often face the same challenge: the need for quality oversight arises long before they are prepared to hire a full-time in-house QA team and/or at a time when they lack the resources to build a Quality Management System (QMS). QMS in a Box is a right-sized, phase-appropriate solution designed for companies that outsource manufacturing and testing. It provides an individualized starter set of Standard Operating Procedures (SOPs), vendor enrollment checklist, executable forms and templates and, when needed, experienced QA resources to implement them.
Developed by experts who have worked in Quality for decades, these SOPs help companies establish the foundation for FDA Phase 1 compliance, vendor qualification, and support the release of phase 1 and 2 clinical supplies. QMS in a Box allows teams to move quickly from preclinical proof-of-concept to GLP toxicology studies and ultimately to their first clinical batch—without losing time or credibility with regulators, investors, or partners.
In this paper, we will take a look at the following:
I. Virtual biopharma company tendencies and associated risks
II. Signs that it’s time to consider QMS in a Box
III. What does phase-appropriate mean?
IV. Included in QMS in a Box
V. How QMS in a Box works in practice
VI. Required steps for implementation of a QMS
VII. Supported modalities/product types
VIII. Benefits to early-stage companies
IX. Limitations/geographic scope
X. Conclusion
I. Virtual biopharma company tendencies and associated risks
QMS in a Box was explicitly designed for virtual sponsor biopharma companies: organizations relying on CDMOs and external labs rather than building in-house manufacturing capabilities. Virtual biopharma companies typically prioritize R&D in the early years, delaying QA infrastructure until the initial public offering (IPO). Doing so without QA consulting services acting as your QA infrastructure courts a high degree of risk, especially in these three areas:
Without a scalable approach, writing SOPs from scratch can take 12–18 months, jeopardizing timelines and investor confidence. QMS in a Box solves this problem with a pre-built, phase-appropriate quality system tailored to the needs of virtual biopharma.
Key advantages include:
II. Signs that it’s time to consider QMS in a Box
Three triggers in particular may signal that it is time to implement this type of support:
By starting early, companies avoid last-minute crises and ensure that SOPs, training, and QA sign-off are in place when critical milestones arrive.
III. What does phase-appropriate mean?
A phase-appropriate quality system describes an approach in which sponsor companies implement procedures and processes that are based on FDA’s Phase 1 GMP guidance but that also takes into consideration regulatory requirements from ICH E6 and 21 CFR 58. This ensures that the appropriate level of oversight is given to CROs, CDMOs and testing lab, and is appropriate for the particular phase of development, such as Phase 1 Clinical Trials.
IV. Included in QMS in a Box
At its core, QMS in a Box is a starter set of SOPs covering essential quality functions across GxP domains. These include:
These SOPs are:
In addition, companies can engage QA resources to execute and manage these systems. BTL and DHC employee numerous experts with the necessary knowledge to act as either:
.png)
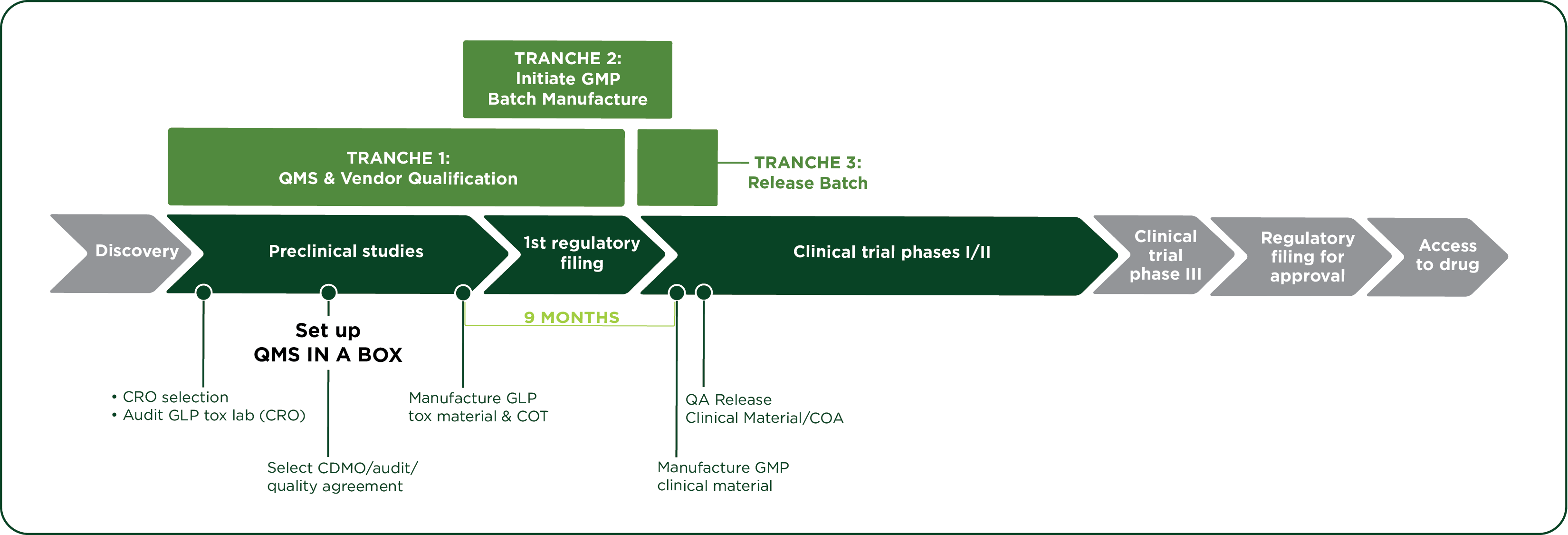
V. How QMS in a Box works in practice
Implementation typically follows a phased approach:
This “tranche” model ensures that each client has the option to build out their Quality systems in lock-step with development, avoiding both under-preparation and over-engineering.
VI. Required steps for implementation of a QMS
In order:
VII. Supported modalities/product types
QMS in a Box can support any modality or product type that DHC or BTL supports. A non-exhaustive list of the most common product types is as follows:
VIII. Benefits to early-stage companies
Benefits to Early-Stage Companies
IX. Limitations/geographic scope
QMS in a Box is designed to ensure compliance with the US FDA’s Phase 1 GMP guidance document. This guidance is unique to the United States, and therefore, so is QMS in a Box.
Therefore, these SOPs are not designed to support European (EMA/MHRA) regulatory environments, where companies must follow directives without direct early-phase guidance. While QMS in a Box can be adapted for global use, this off-the-shelf package is U.S.-specific.
Both BioTechLogic and Dark Horse Consulting offer deep Quality support in all other global markets; the distinction is simply that this level of support requires further customization outside the US.
The regulations that currently exist for governing phase-appropriate GMPs in the US are:
US Law
US Guidance Docs
.png)
X. Conclusion
Every biopharma startup (e.g. sponsor company) faces the same temptation: to delay quality until later. However, ‘later’ usually means ‘too late.’ QMS in a Box bridges the gap by delivering phase-appropriate SOPs and QA support exactly when they’re needed, ensuring that companies are prepared for regulatory expectations, vendor audits, and clinical batch release.
Whether you have QA resources in-house or need outsourced support, QMS in a Box is a shortcut to a compliant, investor-ready quality framework.