
By Stephanie Byrne
Per DSCSA, the U.S. pharmaceutical industry must be exchanging EPCIS data at the serialized package level by November 27, 2023. Recent responses from AmerisourceBergen, Cardinal Health, and McKesson are “fast forwarding” the implementation timeline for manufacturers by requiring serialization data as early as November 2022. There are several options for establishing compliant capabilities and immediate steps that can be taken. Meeting the accelerated deadline is feasible if manufacturers start now.
The Drug Supply Chain Security Act (DSCSA) requires pharmaceutical trading partners to share data that tracks products and provides traceability through the supply chain. The sharing of serialization data relies on Electronic Product Code Information Services (EPCIS), an internationally accepted GS1 standard. EPCIS supports interoperable exchange of serialization data between trading partners.
Today, DSCSA only requires sending lot-level transaction data. However, full traceability requirements mandate EPCIS data exchange at the serialized package level by November 27, 2023. This will require cooperation between manufacturers, wholesale distributors, service providers and other supply chain members. The increased traceability is ultimately intended to enhance U.S. pharmaceutical supply chain security.
AmerisourceBergen, Cardinal Health and McKesson recently addressed these requirements proactively.
Other distributors will likely follow their lead. Specialty pharmacy providers and other dispensers may also begin demanding data in advance of the 2023 enforcement deadline.
Manufacturers must now prepare to provide serialization data to at least Cardinal Health and McKesson by November 2022 – a full year earlier than the DSCSA enforcement date.
For companies using Third-Party Warehousing (3PL) partners, there are several options for establishing compliant capabilities. However, manufacturers will continue bearing the regulatory responsibility to affix serialization data to packages and homogenous cases, send serialization data to customers, store/maintain serialization records, and respond to product verification requests. This responsibility will stay with the manufacturer regardless of who performs packaging, facilitates serialization data exchange, ships physical product, or performs other related activities on behalf of the manufacturer.
Manufacturers may use their own serialization solution and resources to send data downstream to their distributors. This includes capturing data by scanning serialized barcodes and compiling the aggregated data for shipment. This can be accomplished regardless of whether warehouse operations and scanning are performed in-house or at a 3PL. Manufacturers should work with their serialization solution provider to identify and set up the functionality needed to capture and send EPCIS data to distributors if they have not already.
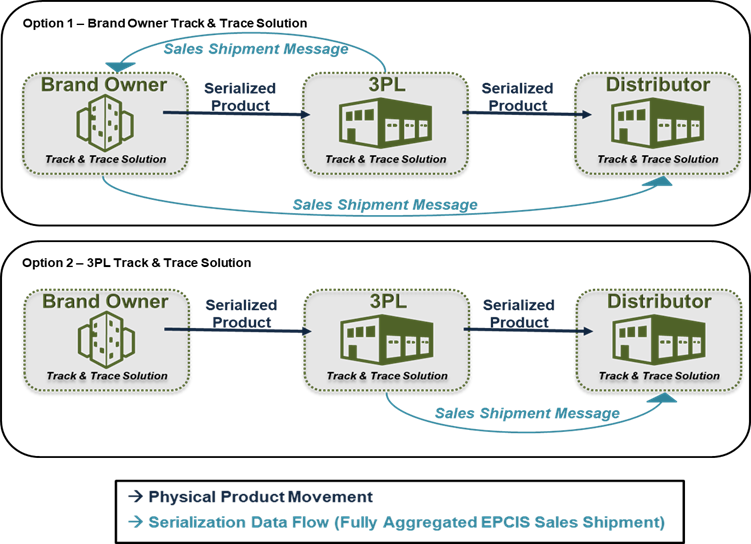
When manufacturers contract with 3PLs for warehousing, logistics, and/or order fulfillment services, the 3PL also performs physical shipment of product to customers. Many 3PLs are establishing capabilities to capture serialization data for shipments during outbound scanning, and depending upon those capabilities, manufacturers may be able to leverage their services to send EPCIS data to distributors.
For a detailed outline of the costs and risks manufacturers should consider while evaluating their 3PL options, check out 2022 Industry Serialization Requirements: Assessing 3PL Options for Sending EPCIS Data to Distributors.
The full traceability requirements stand regardless of whether the manufacturer is already serializing commercial products or is planning a commercial launch in 2022.
To meet Cardinal Health’s and McKesson’s serialization requirements by November 2022, manufacturers should take the following steps now:
On an industry level, these efforts will strengthen the security of the drug distribution supply chain. However, there is limited expertise when it comes to implementing solutions to meet DSCSA requirements. Collaborating with external partners and service providers who have experience evaluating and implementing these solutions can extend manufacturers’ internal bandwidth, promote efficiency and effectiveness in these efforts. It can also provide assurance that the implemented solutions are the right fit for the organization.
For more Supply Chain insights, events, and networking, join Converge’s Supply Chain Working Group and keep the conversation going.
Subscribe to our mailing list for the latest insights on advanced therapy development, regulatory updates, industry trends, and upcoming events from Dark Horse Consulting Group.
We respect your privacy. Unsubscribe at any time. We will never sell your information.