Written by: Kristin Baird, M.D., Master Practice Expert, Eric Humes, RQAP-GLP, Senior Practice Expert, and Matthew A. Spear, M.D., Chief Medical Officer
Introduction
In Part 1 of our Roadmap to FIH, “Charting a Path to Success”, we outlined construction of a first-in-human (FIH) development roadmap as a foundational tool for cell and gene therapy (CGT) programs seeking to secure stakeholder investment and align CMC and nonclinical activities toward IND submission. We described the value-add of a well-designed roadmap and illustrated the “why, when, and how” of roadmap construction for CMC and nonclinical pathways.
In Part 2 of our “Roadmap to FIH” series, we will expand the framework to incorporate a clinical development companion to expedite processes and avoid preventable pitfalls that have delayed many CGT programs despite strong preclinical promise. We will provide an overview of the clinical development pathway, including quality, compliance and risk management considerations while highlighting why strong strategic and regulatory planning and organizational clinical development fitness prior to FIH is essential. Additionally, we will offer guidance on when to build your clinical roadmap and share the DHC approach to applying phase-appropriate strategies and Good Clinical Practice (GCP) principles prior to FIH dosing.
Why build a companion Clinical Development Roadmap?
As discussed in Part 1, the key inflection point for major investment in CGT programs is the generation of positive safety data and early proof-of-concept (PoC) efficacy in patients. Yet across clinical development programs, many Sponsors—despite strong CMC and preclinical foundations supported by significant early investment—experience costly delays once they enter the clinic. These setbacks often stem from inadequate upfront planning or gaps in understanding the critical steps required to support a successful clinical launch. As a result, development timelines can be extended by months or even years when early-phase studies are operationally under-planned, lack quality systems ensuring reliability of results and data integrity, or are not executed in alignment with the Sponsor’s regulatory obligations, which can lead to slow enrollment, regulatory delays, or unexpected safety or efficacy results.
Beyond defining key timelines and inflection points, a well-constructed clinical development roadmap enables Sponsors to thoughtfully balance internal capabilities with external support to optimize both cost and speed. For example, selectively outsourcing clinical leadership—such as engaging fractional and/or embedded Chief Medical Officer (CMO), clinical operations, QA, translational medicine, biostatistics and/or regulatory leads—can accelerate execution and reduce near-term operational costs until the role is justified internally. Conversely, building in-house capabilities may require greater upfront investment and longer ramp-up periods, but can deliver long-term efficiencies and strategic value. A central objective of the roadmap is to clearly identify which activities are required, when they are needed, and whether they represent ongoing or episodic demands over the course of development.
In practice, many clinical leadership roles (e.g., CMO, Head of Clinical Operations, Head of Regulatory) do not need to be filled at program inception and can be phased in as clinical activities scale following key preclinical and CMC milestones. This level of planning is critical for informing insourcing versus outsourcing decisions and for guiding the timing and nature of strategic clinical hires. An important consideration is whether a given capability is readily available through external providers—such as regulatory support, quality and risk management, clinical operations, or supply chain—or whether it represents a highly specialized function that should be internalized to strengthen the company’s competitive advantage.
As noted in Part 1, the rise of the virtual biotech model—lean executive teams relying heavily on consultants and contract organizations—continues to shape the industry. Success with this model depends on a robust and realistic roadmap that integrates accurate cost projections, a clear assessment of program risks, and identification of opportunities to accelerate development. At DHCG, we leverage the collective experience of our team across academia, biotech, and regulatory agencies, along with insights gained from supporting more than 500 CGT clients, to develop tailored clinical development strategies aligned to each program’s unique design space. Our Group will bring in a team that not only has CGT experience and expertise but also has worked together and can quickly integrate with the Sponsor and hit the ground at a gallop.
When Is the Right Time to Build a Clinical Development Roadmap?
A companion clinical development roadmap should begin once the lead candidate product has been identified. The roadmap should be anchored in a clear and well-defined Target Product Profile (TPP), which informs first-in-human (FIH) study design and the selection of the initial patient population. Once these foundational elements are established, the clinical development roadmap can be built in a structured and intentional manner.
Building a Clinical Development Roadmap for Success
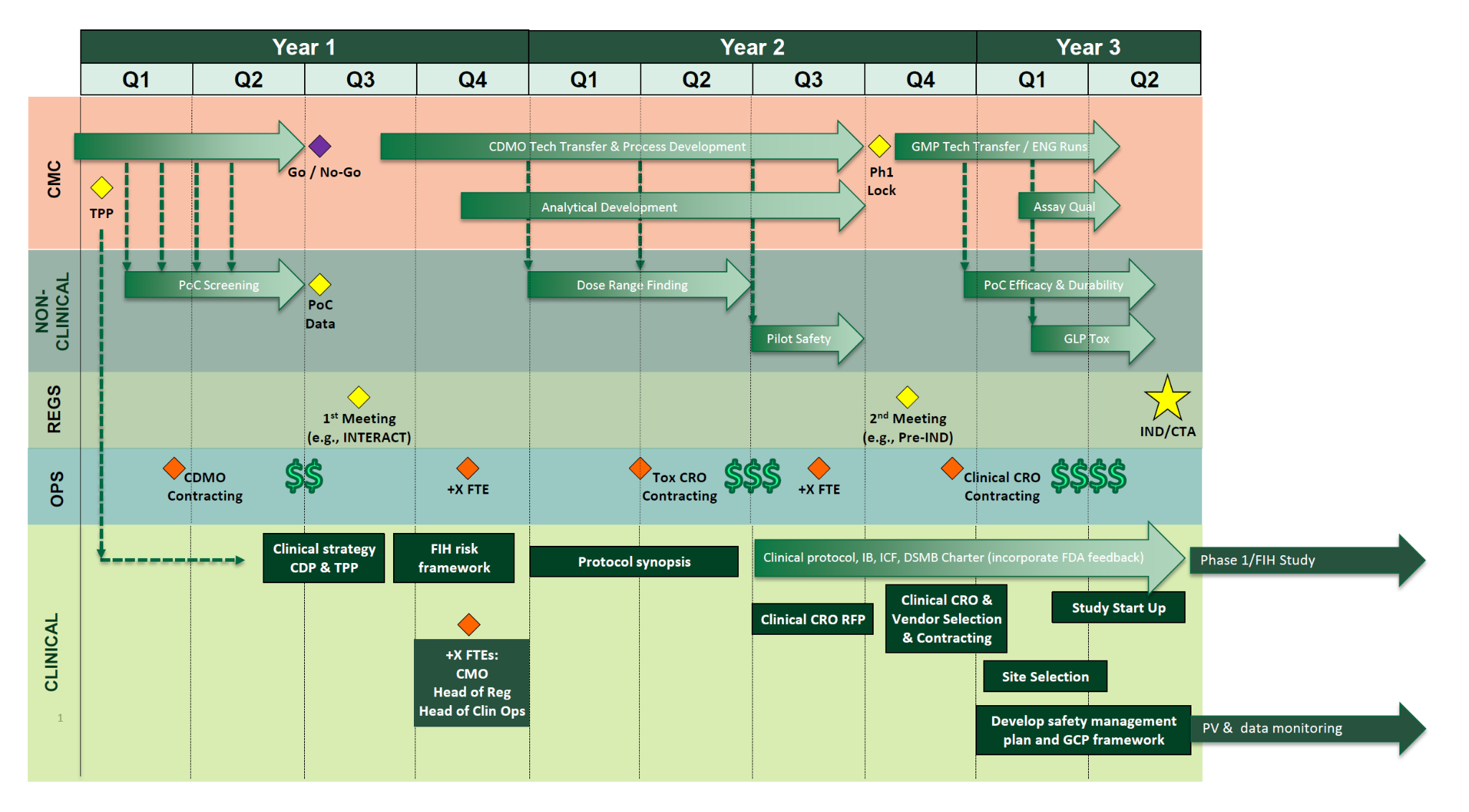
A robust clinical development roadmap outlines the regulatory, logistical, and operational components required to advance a program efficiently and effectively. The overall timeline should be informed by key preclinical and manufacturing milestones, with alignment of regulatory interactions to support overall program progression.
Once a target IND submission date is identified, clinical development timelines can be reverse-engineered to ensure readiness. A representative sample timeline is shown in the figure above, with key components described below.
Clinical Development Strategy & Indication Selection
Target Product Profile (TPP) and Patient Population Rationale
The Target Product Profile (TPP) is a strategic planning document that defines the desired attributes of a product and guides development over time. It aligns early preclinical activities with long-term regulatory, clinical, and commercial objectives.
Core components of a TPP typically include:
TPPs are often structured with “minimal” and “optimal” targets for each attribute to guide decision-making as data mature. Defining the ultimate goals of the clinical program early enables more effective and proactive strategic development.
Additional early considerations include disease biology, unmet medical need, standard of care, and endpoint landscape, all of which inform clinical strategy and regulatory engagement throughout the CGT product development lifecycle.
FIH Translation & Risk Framework
A structured translation and risk framework should be developed to inform a well-designed FIH Protocol Synopsis, which is the initial summary section of the clinical trial protocol and captures the critical elements required for conducting the clinical trial. These include:
Similar to a CMC control strategy, the FIH Protocol Synopsis serves as a study-specific “control document.” It drives development of the full clinical protocol while ensuring clear, unambiguous eligibility criteria and operational feasibility.
The synopsis also establishes the foundation for:
As such, the FIH Protocol Synopsis is a key building block in ensuring the clinical development roadmap is sufficiently detailed, actionable, and execution-ready.
Operational Readiness & FDA Interactions
Key activities to support operational readiness include:
CRO selection is distinct from a Clinical Quality Assurance (CQA) audit. Clinical Operations and Clinical Development subject matter experts should define outsourcing criteria based on the finalized FIH Protocol Synopsis and determine which regulated activities will be delegated to CRO partners.
Once selected, Sponsors must establish a clear and effective oversight and governance model. While CROs may assume responsibility for investigator and monitor selection, and subsequently enrollment, data collection, and site monitoring, the Sponsor’s clinical operations and development teams retain ultimate accountability for oversight and decision-making.
Authoring of Core IND Clinical Documents
DSMB Planning & Governance
IND Submission and 30-Day Review Activities
DHCG Approach
At DHCG, we apply a thoughtful, analytic approach to establish documented, phase-appropriate, and fit-for-purpose clinical development strategies, plans, and roadmaps. Our process identifies gaps and development requirements based on current industry best practices, therapeutic modality–specific considerations, and evolving regulatory expectations for CGT drug products.
In the clinical development context, this means establishing a framework that supports future operations - from initial patient population and indication selection and safety and risk management planning to the development of high-quality protocol documents optimized for signal detection, operational efficiency, and high-integrity data collection.
DHC can support these activities through:
Conclusion
A well-constructed clinical development roadmap is essential for CGT Sponsors preparing for a first-in-human (FIH) clinical trial. Demonstrating safety and efficacy during PoC in the target study population has become one of the primary determinants of overall clinical development success. An effective roadmap enables lean management teams to focus on the most critical elements of the investigation plan while demonstrating control over critical-to-quality risk factors. The clinical development roadmap serves as a strategic counterpart to the CMC and nonclinical roadmaps, supporting internal decision-making, strengthening investor confidence, guiding vendor strategy, and establishing a strong foundation for successful clinical program execution from FIH study launch to Phase 3 through BLA.
Be on the lookout for Part 3 of our series, Roadmap from FIH to BLA, where DHC experts will explore key considerations for planning the next phase of product and clinical development, offering practical insights into navigating the path from FIH to BLA.